Research
Medical Device Industry: Key Points to Remember
What are Spine Biomechanics?
Spine biomechanics (as well as biomechanics as a whole) studies the mechanical behavior of the spine (and other musculoskeletal structures) under various loads and conditions. I found the YouTube video below informative, not only with respect to structure but also other essential aspects, such as neurology, pathology, and relevant complications.
https://www.youtube.com/watch?v=ofiTvFWkuLM
The Article I linked to below was very informative, as it gave some pushback against my "Design Philosophy". The truth of the matter is, some companies verify and validate the requirements for their devices differently from others. This can occur through non-traditional testing methods (a new, undocumented DOE operating through back channels) or close collaboration with the FDA, which then influences others' approaches. Reading this early last year made me realize data is not as objective as I thought.
https://pmc.ncbi.nlm.nih.gov/articles/PMC4365606/
When beginning my orthopedics 3D-printing journey, the article below also helped me find my way when it came to material selection, what to expect with FDM, as well as insights into what to expect in the rest of my career:
510(k) Submissions are essential to ensuring a product is used safely and effectively in the real world. Although I have collected data and essentially performed traceability on the recent work I did with V&V, the article below helped me understand the rest of the submission process, particularly with respect to the post-market aspect.
Relevant Standards to Remember
FDA, ISO, and ASTM standards relevant to spine:
Pre-op Evaluation
Preoperative evaluation assesses a patient's readiness for spinal surgery. In a macro sense, different engineering companies approach this differently. Some use a data-driven approach when it comes to navigation and a standard product line. I am fond of the patient-specific approach, which I believe will gain popularity in the future.
Planning iteratively with surgeons based on patient CT scans
Designing an implant in accordance with segmentation and anatomical constraints.
Manufacturing the product in-house in accordance with the patient's timeline.
Sterilization and post-processing to prepare for surgical or clinical use.
This patient-specific approach is currently more expensive than profitable; yet, as surgical outcomes continue to demonstrate an efficiency uptick, I believe the finances will take care of themselves. Seeing this vision at a young age motivates me to work in this industry, and is why I have been preparing a little over a year with my own venture/initative in CTLS.
Post-Op Care
Post-op care involves monitoring recovery and managing pain after surgery. It is essential to be vigilant and follow up with patients regularly after their surgeries to not only be amicable but also detect any post-op surgical complications quickly. This is most applicable in patient-specific applications, where products are tailored specifically to a patient's anatomy.
Biomechanical Research
When it comes to biomechanical research that drives advancements in this field, it is essential to utilize established methodologies, ethical guidelines, and state-of-the-art equipment for data collection and analysis. For example, I found new research at Arthrex interesting:
https://journals.sagepub.com/doi/10.1177/23259671251356627
https://journals.sagepub.com/doi/full/10.1177/23259671251353757
These two articles on Arthrex's biomechanical research were very eye-opening to me, not only because of the experimentation done on the joints, but also in the data analysis section. I gained experience this past year with static testing, but seeing both static and dynamic testing provide different insights into displacement, as well as utilizing different test equipment, was exciting. For example, in the first article on transosseous quadriceps tendons, the comparison of displacements using the optical camera was fascinating, even for such a slight difference in sutures.
In the second article on tibial eminence avulsion fractures, the use of dynamic biomechanical data to get insight into the deviation of cycles was also very insightful.
In both of these cases, the markets are relatively small compared to other areas of orthopedics, yet the culture of innovation thrives. That, in a nutshell, is what I want to be part of moving forward: patient-specific innovation that helps others, regardless of the market size.
Perioperative Procedures
During the surgery (perioperative operations), it is essential to be as prepared as possible when working with surgeons. That means being prepared for both success and failure:
When attending surgeries, engineers need to be prepared and able to provide product expertise to surgeons, not only as a representative would, but also to offer real-time feedback. This means explaining the form and fit of an implant, as well as the reasoning behind it, quickly.
Engineers also need to be prepared to make quick adjustments. This means being ready to present a range of instrumentation choices (e.g., various pedicle screw sizes, pitches, etc.) and explain the rationale behind each. Every surgeon is unique in their preferences, especially when it comes to more complex cases. Playing devil's advocate in situations where expectations are unclear, beginning every case with clear communication leads to better outcomes and ultimately better patient care.
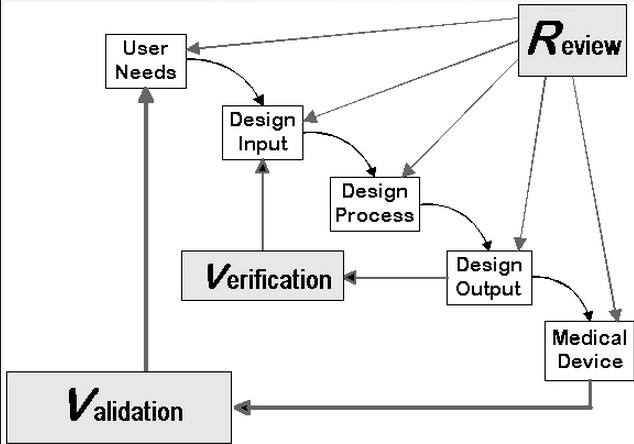
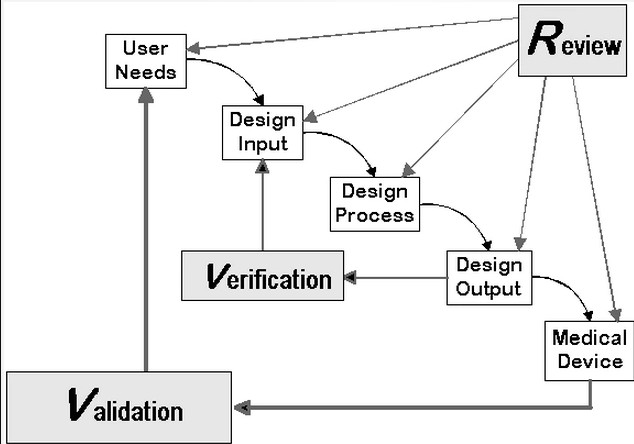
Design Control Application
When it comes to following the design process, setting forth system requirements, conducting design reviews at each step, and ultimately completing verification and validation in the medical device industry, this is the graphic I keep in mind. This aligns with my design philosophy, which prioritizes the user's needs and safety.
